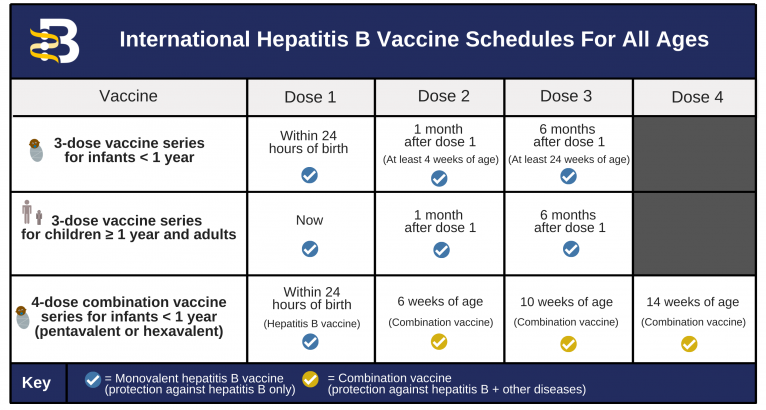
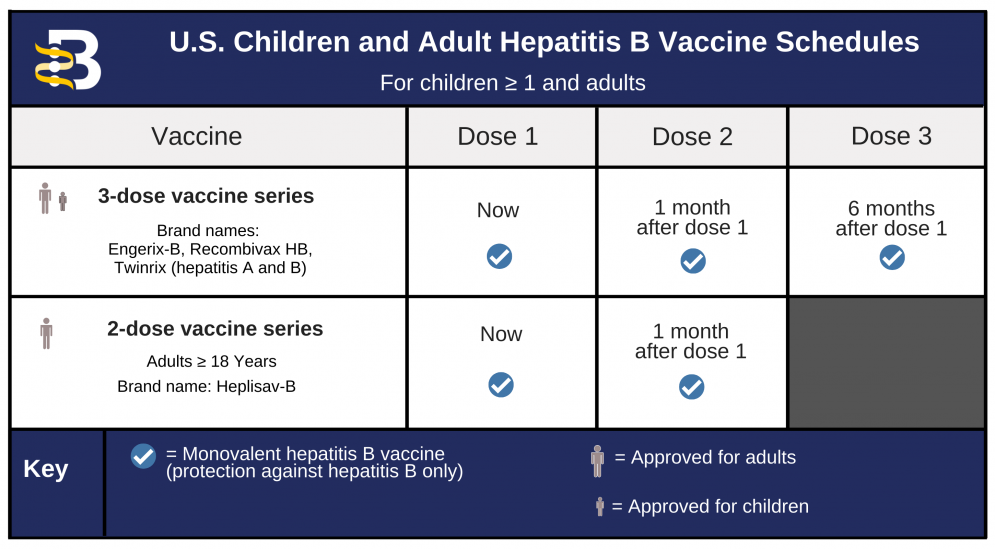
According to Imelda Garcia the Associate Commissioner of. Register online at GetTheVaccinedshstexasgov.
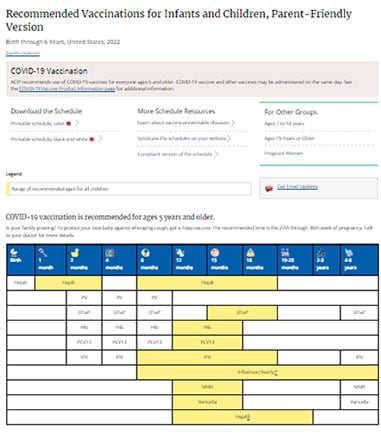 Recommended Immunization Schedules For Children And Adults
Recommended Immunization Schedules For Children And Adults
By Appointment ONLY Immunization records available for.

Texas vaccine records. The State of Texas maintains an immunization registry --ImmTrac2-- that can provide immunization records for areas in Texas other than Tarrant County. Call 2-1-1 to find local vaccine providers and learn what forms of ID are accepted. All doctors have access.
The state requires vaccine providers to enter COVID-19 vaccine data into the states immunization registry within 24 hours of administering a vaccine according to. Records may also be obtained through the Texas Immunization Registry. 032017 Use this form to authorize the release of your or your childs ImmTrac2 records.
No matter how many times you move or change health care providers your and your familys immunization records are at any Texas doctors fingertips. If you need proof of immunization for a child or adult start here for an explanation of how to obtain your records from lmmTrac. Schools can verify vaccine records.
Eastside Clinic 210 Mel Waiters Way San Antonio TX 78202 2102078894 Open. Monday - Friday for Immunization Records and Vaccines 800 am. The cost for immunization records is 500.
Learn more about immunizations records. The new Texas Vaccine Scheduler helps Texans get scheduled for a COVID-19 vaccine at clinics hosted by participating Texas public health entities. Since 2005 Texas law has required that all healthcare providers report to lmmTrac all vaccines administered to children younger than 18 years of age.
To make it easier to schedule a vaccination the Department of State Health Services DSHS has launched a new statewide toolthe Texas Public Health Vaccine Schedulerto help anyone age 16 or older schedule a vaccination in their area. Texas will receive more than 25 million doses of the COVID-19 vaccine next week a record number of doses state health officials said Friday. Keeping up with vaccine records is now easier than ever thanks to ImmTrac2 the Texas Immunization Registry.
To request an ImmTrac immunization record contact. HttpstcoXptNvbu24U Keya Vakil keyavakil March 2 2021 Abbott it turns out wasnt the only governor to ease restrictions Tuesday Mississippi Gov. You will be notified by email or text when and where to get the vaccine.
Tate Reeves R actually beat him to the punch announcing that businesses can operate at full capacity and county. Texas Public Health Vaccine Scheduler. The Texas Immunization Registry makes it easy to keep up with your and your familys immunization records.
The first side-effect case from the Johnson Johnson COVID vaccine has been recorded in Texas. Texas Department of State Health Services DSHS offers the Texas Immunization Registry at no cost to all Texans. Try looking through baby books or other saved documents from your childhood.
They can be reached at 210 207-8894. Ask parents or other caregivers if they have records of your childhood immunizations. Administrative Office - 2102078790 Clinic.
If you need official copies of vaccination records or if you need to update your personal records there are several places you can look. Back to Public Health Main Page. Parents or guardians can also choose a religious exemption from immunization requirements.
Texas registry of critical vaccine information needs a boost When children reach adulthood they must opt-in to the Texas registry and this process creates confusion. Since 2005 Texas law has required that all healthcare providers report to the Texas Immunization Registry all vaccines administered to children younger than 18 years of age. Flu Vaccine Information Learn where to get a flu shot how to prevent getting the flu and information.
Request and Immunization Record. The registry is secure and confidential and safely consolidates and stores immunization records from multiple sources in one centralized system. To request a copy of immunization records for a child under 18 years of age please complete and submit by fax or mail an Authorization to Release ImmTrac2 History Form F11-11406.
We do this through administration of the Texas Immunization Registry ImmTrac2 which provides access to immunization records establishment of school immunization rules and administration of the Texas Vaccines for Children and Adult Safety Net programs which provide low-cost vaccines to eligible children and adults. Texas law allows physicians to write medical exemption statements for vaccines that would be medically harmful or injurious to the health and well-being of the child or a household member. F11-11406 Immunization Registry ImmTrac2 Authorization to Release Official Immunization History rev.
Texas is bottom 5 in per capita vaccination rates yet the governor seems to believe the pandemic is over.