Food Allergy Research Education FARE works on behalf of the 32 million Americans who have food allergies including all those at risk for life-threatening anaphylaxis. 20200304_FARE_EG_0604 by Food Allergy Research Education 20200304_FARE_EG_0585 by Food Allergy Research Education 20200303_FARE_EG_0515 by Food Allergy Research Education.
 Epidemic Infographic Food Allergy Research Education
Epidemic Infographic Food Allergy Research Education
Food Allergy Research Education receives 8721 out of 100 for their Charity Navigator rating.

Food allergy research & education. Help Us Accelerate Food Allergy Research Join over 10000 individuals and families managing food allergies who are sharing their food allergy stories and making a critical difference helping to speed the search for new treatments and informing life-changing improvements in patient care. Or roughly two in every classroom. Food Allergy Research Education is rated 3 out of 4 stars by Charity Navigator.
The picture widens to nearly 85 million Americans impacted when you account for all the people who shop for cook for and live with those with severe food allergies as well as those with food intolerances and avoidances. The goal of this study is to establish a birth cohort that collects prenatal and early life biosamples and environmental samples and rigorously phenotypes young children for food allergy and Atopic Dermatitis AD to identify prenatal and early life markers of high risk for food allergy and AD as well as biological pathways endotypes that result in these conditions. Food Allergy Research Education FARE is the leading nonprofit organization working on behalf of the 15 million Americans with food allergy including all those at risk for life-threatening anaphylaxis.
FARE Food Allergy Research Education is the worlds leading non-governmental organization engaged in food allergy advocacy and the largest private funder of food allergy research. The organization is run by Lisa Gable and has an annual revenue of 14566688. Our mission is to improve the quality of life and the health of individuals with food allergies and to provide them hope through the promise of new treatments.
Food Allergy Research Education FARE works on behalf of the 15 million Americans with food allergies including all those at risk for life-threatening anaphylaxis. Food Allergy Awareness Week Over 32 million Americans have a serious and potentially life-threatening food allergy. Food Allergy Research Education organized the Oral Immunotherapy for Food Allergy Summit on November 6 2019 modeled after the PRACTALL sessions between the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy Asthma Immunology to address these critical issues.
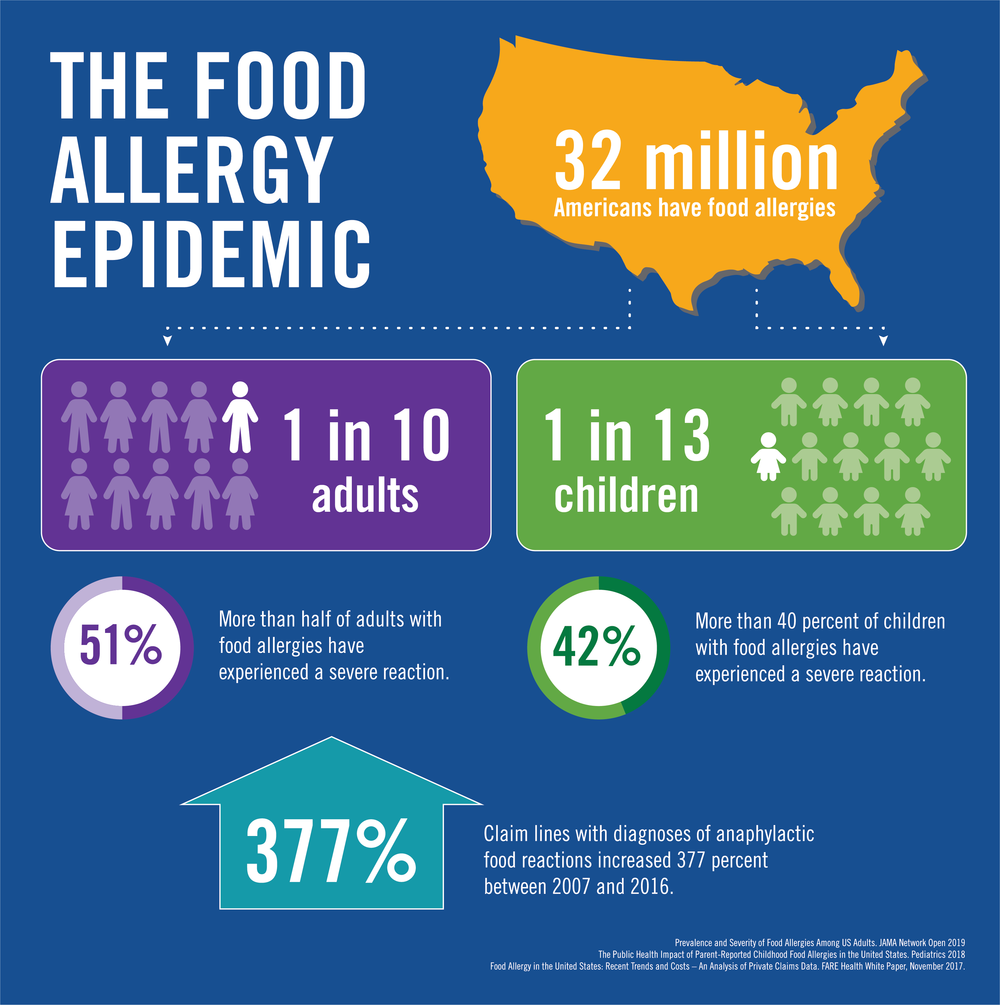
This potentially deadly disease affects 1 in every 13 children in the USA. FARE works on behalf of the 32 million Americans who have food allergies including all. Food allergy FA is a serious public health concern that causes potentially-life threatening reactions in affected patients.
Food Allergy Research Education McLean VA. The Registry is sponsored by FARE Food Allergy Research Education which works on behalf of the 32 million Americans who have food allergies. House has approved the Food Allergy Safety Treatment Education and Research FASTER Act bringing sesame one step closer to becoming the ninth major allergen as defined by federal law.
The prevalence of food allergy in the United States US has increased substantially and now affects 15 million patients4-8 of children 6 million children 30 with multiple food allergies and about 9 of adults. Food Allergy Research Education is a Medical Research charity located in McLean VA. In fact the chief executive officer of the organization Food Allergy Research and Education recently described the availability of mental health and other supportive care resources to individuals with food allergies as totally inadequate and the United States as severely lacking in therapists who have studied and understand food allergy Baker 2017 p.