Researchers including research study staff and students working with human subjects or data and samples from humans must sometimes complete training in human subjects protections in order to meet the requirements of the organizations they are affiliated with or of funding organizations. Human Subjects Research HSR content is organized into two tracks.
 Instructions For Completing Human Subjects Research Training Office Of Research Support And Compliance
Instructions For Completing Human Subjects Research Training Office Of Research Support And Compliance
Institutional and Signatory Officials.
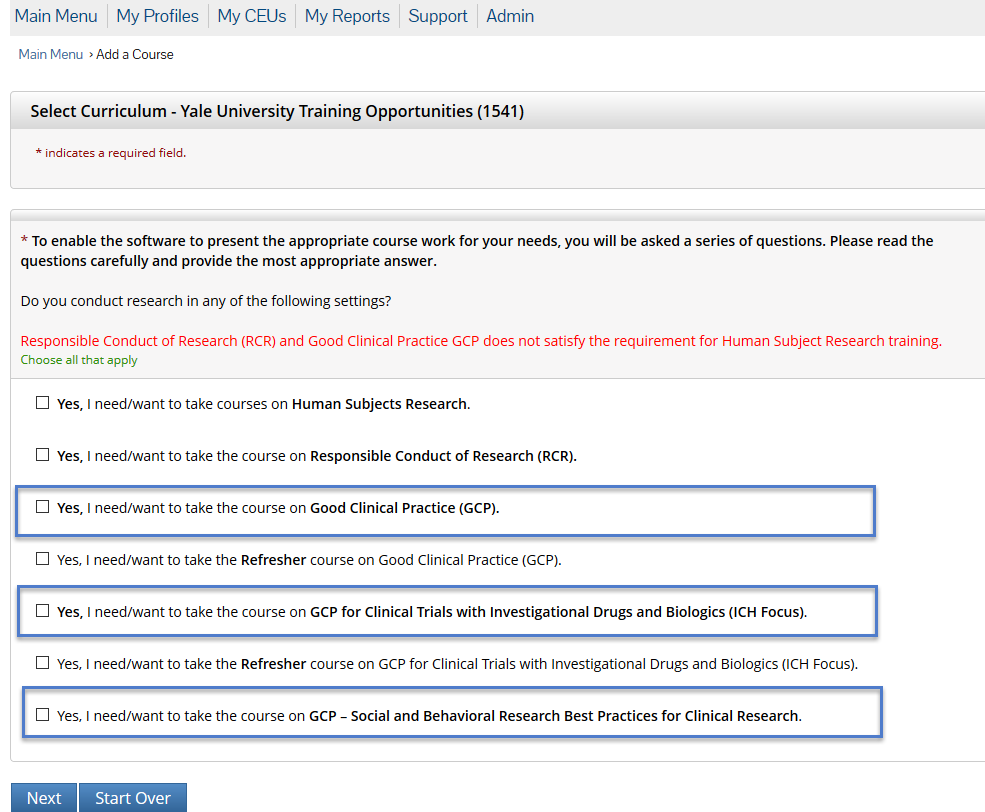
Human subjects research training. Complete all enrollment information. A printable completion certificate is available at the conclusion of the lesson so viewers can document completion for their records. Training Requirements for Human Subjects Protection The Veterans Health Administration VHA Office of Research and Development ORD requires all individuals involved in conducting VA human subjects research to complete training in the ethical principles on which human subjects research is to be conducted no less frequently than every 3 years.
If you engage in Biomedical or Social and Behavioral research then you must complete a specially designed web based training course by following the link to the University of Miami CITI program. CSULB uses the CITI Program to provide trainings for Human Subjects and Animal Welfare research Financial Conflicts of Interest and to fulfill federal Responsible Conduct of Research RCR requirements. Find useful information about proposing and conducting NIH extramural research involving human subjects including policies regulations training and resources.
Human Subject Protection Staff. Human Subjects Research Training SOP Purpose. Research Ethics and Compliance Training CITI Program.
Institutional Review Board Members. NIH-funded domestic and international grantees conducting human subjects research must comply with the NIH. To ensure that grantees comply with the NIH mandate to take training in the protection of human subjects.
Biomedical Biomed and Social-Behavioral-Educational SBE. Human Subjects Research Conflict of Interest COI Good Clinical Practice GCP and other Institutional Compliance Training HIPAA Training on Safety of Children Biosafety etc. HSR Biomed and SBE courses are offered as.
One way to achieve this is by completing the Human Research Protection Training offered by the HHS Office for Human Research Protections OHRP. Mandatory Training for Conducting Research on Human Subjects CDCATSDR investigators supervisors Associate Directors for Science ADS and Human Subjects Contacts HSC are required to maintain competency in research ethics and human research regulations by completing specific trainings on a regular basis. Learn about considerations for human subjects research when planning and submitting a research application or contract proposal and throughout the extramural funding cycle.
Human Research Protection Training OHRP offers this comprehensive training for the research community on human research protections based on the requirements of the revised Common Rule or the 2018 Requirements. The Collaborative Institutional Training Initiative CITI Program is dedicated to serving the training needs of colleges and universities healthcare institutions technology and research organizations and governmental agencies as they foster integrity and professional advancement of. They are intended for anyone involved in research studies with human subjects or who have responsibilities for setting policies and procedures with respect to such research including Institutional Review Boards IRBs.
IRB requires all individuals involved in human subjects research to complete training in human subjects protection via the Collaborative Institutional Training Initiative CITI Program which must be completed by both the Principal Investigator and all study staff listed on. Human Subjects Research Training All SU researchers and advisers must complete the CITI Human Subjects Research training program scroll down for details and include current completion certification with expedited and full-board protocol submissions. Each of the RCR course offerings covers the core norms principles and rules governing the practice of responsible research.
The Trusted Standard in Research Ethics and Compliance Training. Select George Mason University from the Participating Institutions drop down menu. Human Subjects Training All investigators faculty staff and students are required to complete the CITI Program training in human subjects protection prior to conducting research using human subjects.
You do not need to submit training certification with applications for exemption. All personnel involved in studies utilizing humans as research subjects must undergo recertification in human subjects research training every three years from the date of original approval. When documents are reviewed by the IRB they are declared as expedited.
Investigators and all key personnel who will be involved in the design or conduct of NIH-funded human subjects research must fulfill the protection of human subjects education requirement. IRB Chairs Administrators and Staff. CITI HUMAN SUBJECT RESEARCH TRAINING 4 The Institutional Review Board IRB is an administrative body established to protect the rights and welfare of human research subjects recruited to participate in research activities conducted under the auspices of the institution with which it is affiliated Watson 2017.
PHRP Online Training is perfect for anyone who will be engaged with human subject research including.











:max_bytes(150000):strip_icc()/3132731_color2-5ba53e0a46e0fb002594fec7.png)
















